26+ orbital diagram for boron
In an orbital energy level diagram individual orbitals are usually represented by horizontal lines whose vertical position conveys the qualitative relative energies of the orbitals. Web A molecular orbital diagram or MO diagram is a qualitative descriptive tool explaining chemical bonding in molecules in terms of molecular orbital theory in general and the linear combination of atomic orbitals LCAO molecular orbital method in particularElectron Configuration for Boron BElectron Configuration for Boron B.
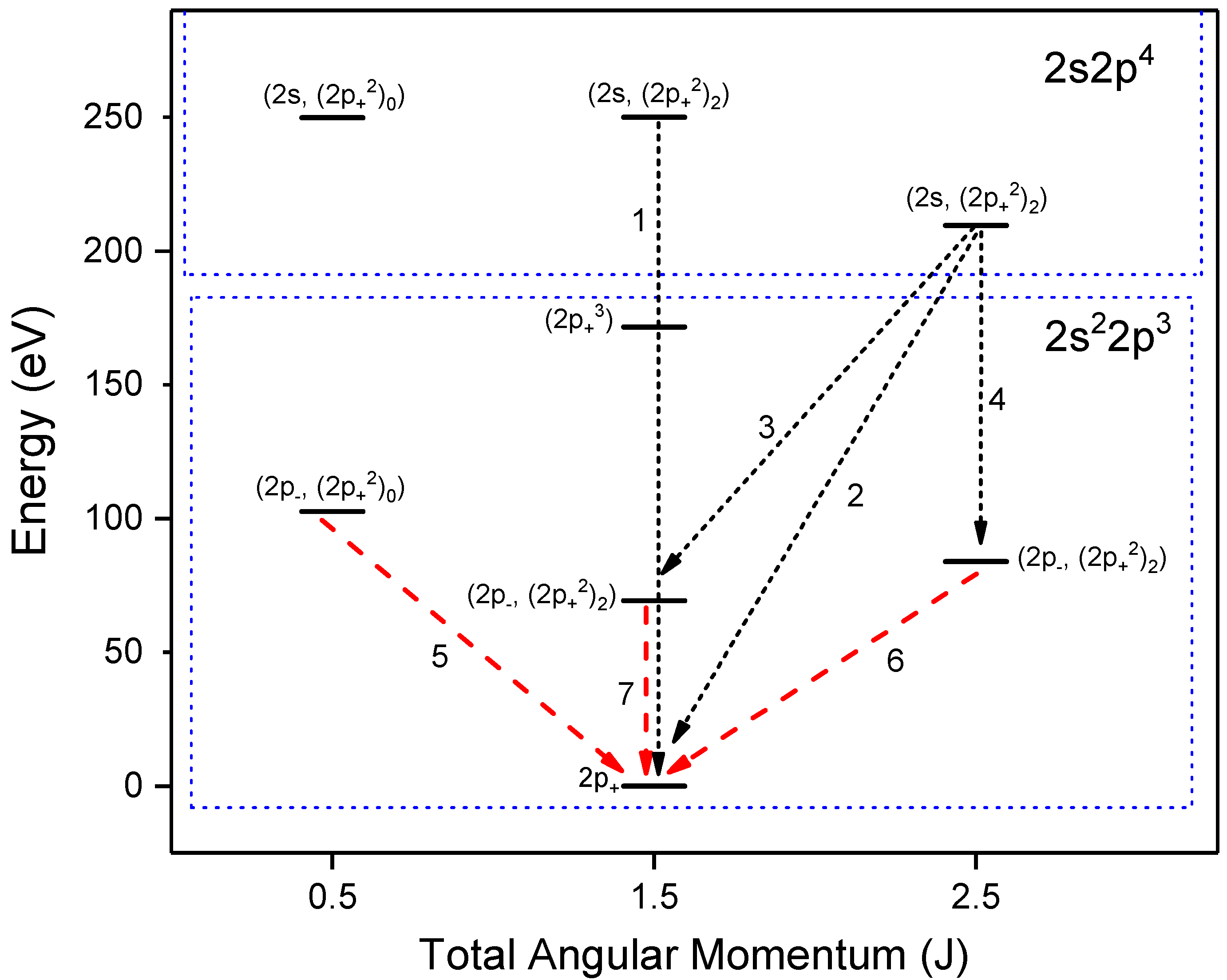
Atoms Free Full Text Identification And Plasma Diagnostics Study Of Extreme Ultraviolet Transitions In Highly Charged Yttrium
The orbital diagram for beryllium is shown here.

. Web Orbital diagrams are pictorial representations of the electron configuration showing the individual orbitals and the pairing arrangement of electrons. 1s orbital contains 1 box 2s orbital also contains 1 box and 2p orbital contains 3 boxes. The most important compounds of boron are boric or boracic acid.
Labels can be used once more than once or not at all. Steps 1 Find Electrons of Boron 2 Write Electron Configuration of Boron 3 Draw Orbital Diagram of Boron Steps Heres how you can draw the orbital diagram of boron step by step. The next element is beryllium which has four electrons.
Boron has 3 valence electrons and nitrogen has 5 valence electrons this makes 8 electrons. Web The orbital diagram of boron shows that the 1s subshell has 2 electrons the 2s subshell has 2 electrons and the 2p subshell has 1 electron. The electron configuration is 1s 2 2s 2.
A H and H 2 b N and N 2 c O and O 2 d C and C 2 e B and B 2. Web Orbital Diagram of all Elements 118 Orbital Diagrams Inside Orbital Diagram of all Elements 118 Orbital Diagrams Inside Orbital diagrams orbital box diagrams for all elements of periodic table are shown in. Web Boron is the fifth element with a total of 5 electrons.
We start with a single hydrogen atom atomic number 1 which consists of one proton and one electron. Web The orbital diagram of Boron contains 1s orbital 2s orbital and 2p orbital. We start with a single hydrogen atom atomic number 1 which consists of one proton and one electron.
We already know that the p-sub shell has three orbitals. Web Orbital diagrams are pictorial representations of the electron configuration showing the individual orbitals and the pairing arrangement of electrons. The ground state electron configuration of boron is 1s 2 2s 2 2p 1.
This is the general MO diagram you need to fill with the valence electrons of BN. This is called quantum jump. Since 1s can only hold two electrons the next 2 electrons for B goes in the 2s orbital.
Web The energy level diagram on the left shows the relative energy of the 2s and 2p orbitals based on the ability of the sublevels to penetrate to the nucleus. Web To write the orbital diagram for the Boron atom B first we need to write the electron configuration for just B. Web Pure boron is a dark amorphous powder.
In writing the electron configuration for Boron the first two electrons will go in the 1s orbital. Drag the appropriate labels to their respective targets. You have to start filling the orbitals from those with lowest energy to those with higher energy.
Web Orbital Diagram for Boron Electron configuration of boron in the excited state Atoms can jump from one orbital to another in an excited state. Web Compare the atomic and molecular orbital diagrams to identify the member of each of the following pairs that has the highest first ionization energy the most tightly bound electron in the gas phase. Complete an orbital diagram for boron.
Web An orbital filling diagram is the more visual way to represent the arrangement of all the electrons in a particular atom. In an orbital filling diagram the individual orbitals are shown as circles or squares and orbitals within a sublevel are drawn next to each other horizontally. Web An orbital energy level diagram or just orbital diagram shows the relative energies of orbitals and how electrons are distributed among orbitals within a subshell.
Chemistry questions and answers. Web This video discusses how to draw the molecular orbital MO diagram for the B2 boron molecule. Web Solved Complete an orbital diagram for boron.
Boron has a total of 5 electrons and one box can hold up to the two electrons. Complete an orbital diagram for scandium Sc. Uses Amorphous boron is used as a rocket fuel igniter and in pyrotechnic flares.
To do that we need to find the number of electrons for the B atom there are. Web 1 Answer. The bond order of the boron molecule is also calculated and the meaning of this number is.
It gives the flares a distinctive green colour.
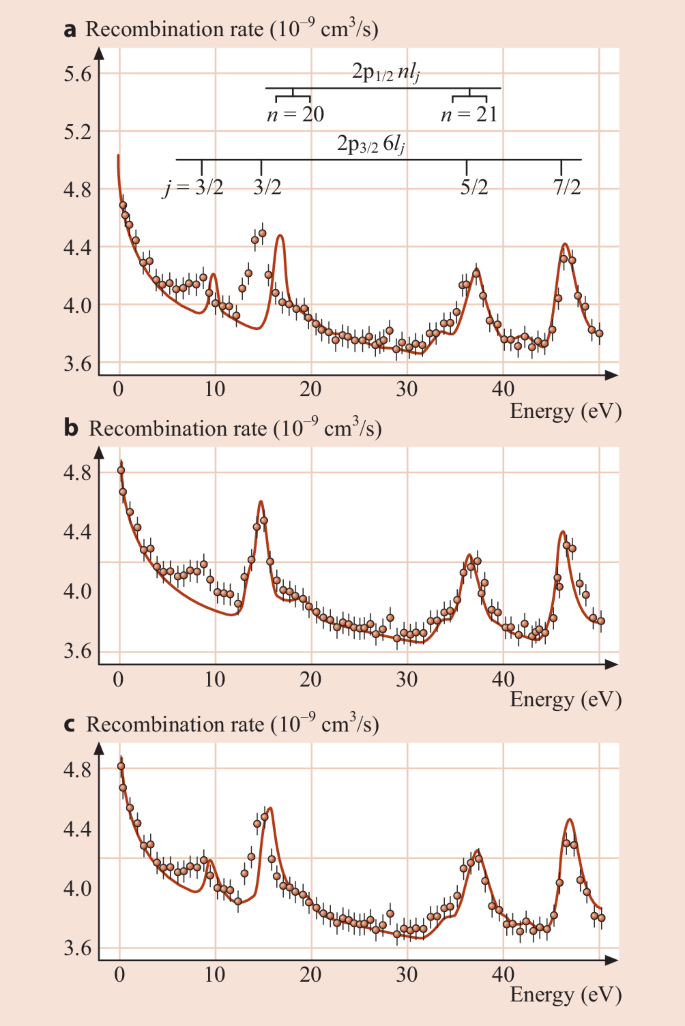
Dielectronic Recombination Springerlink

Emission Of X Rays In Collisions Of Xenon Ions With Metal Surfaces Sciencedirect

Platinum Complexes Of Terpyridine Interaction And Reactivity With Biomolecules Sciencedirect

Synthesis And Coordination Chemistry Of A Benzannulated Bipyridine 6 6 Biphenanthridine Inorganic Chemistry

Synthesis And Coordination Chemistry Of A Benzannulated Bipyridine 6 6 Biphenanthridine Inorganic Chemistry

Interactions Of Metal Based And Ligand Based Electronic Spins In Neutral Tripyrrindione P Dimers Inorganic Chemistry
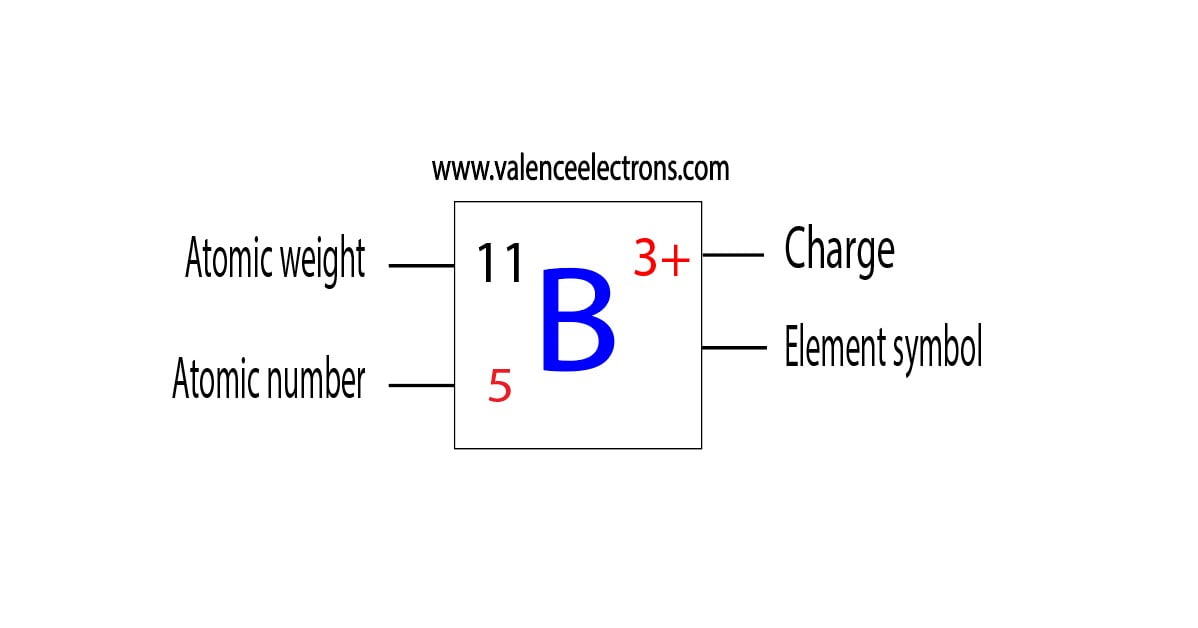
Boron B Electron Configuration And Orbital Diagram

Platinum Complexes Of Terpyridine Interaction And Reactivity With Biomolecules Sciencedirect

Emission Of X Rays In Collisions Of Xenon Ions With Metal Surfaces Sciencedirect

Electron Configuration Of Boron Smartcityvaranasi

A Hartree Fock Orbital Diagram Showing The Energy Of The Download Scientific Diagram

Electron Configuration Of Boron Smartcityvaranasi

Boron B Electron Configuration And Orbital Diagram
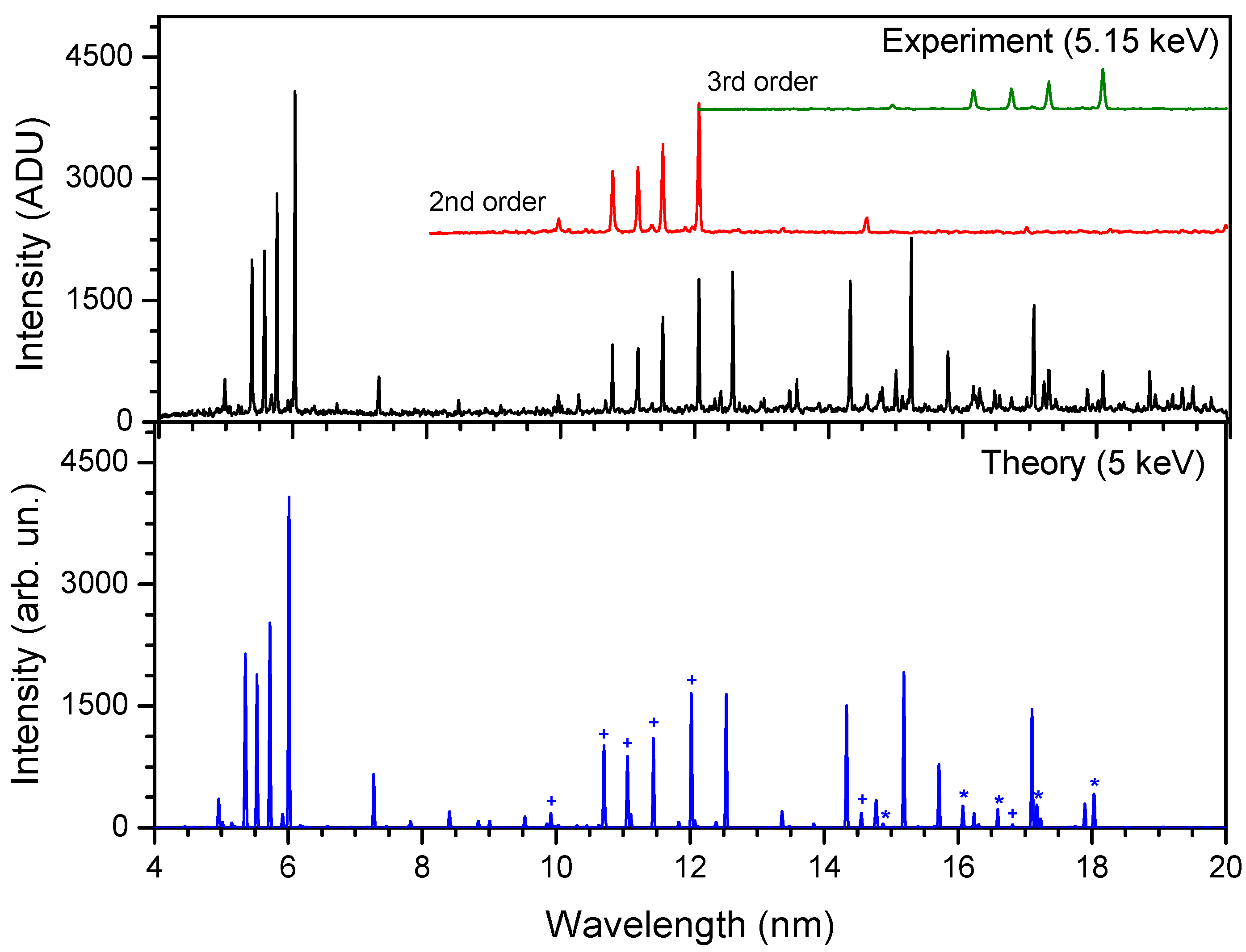
Atoms Free Full Text Identification And Plasma Diagnostics Study Of Extreme Ultraviolet Transitions In Highly Charged Yttrium

Boron Electron Configuration Youtube
![]()
Boron Electron Valence Borates Today

Monomeric And Trimeric Thorium Chlorides Isolated From Acidic Aqueous Solution Inorganic Chemistry